How to Kill Your Imaginary Friends: Superballs, Pockets, and Fun With Awesome Molecules
By Dr. Grasshopper
Dear Dr. Grasshopper,
I’m writing a military sci-fi novel, and I’ve run into a medical snag.
I know (or like to think) that there are certain toxins which can rob the blood’s ability to transport oxygen. Would you happen to know what the emergency treatment is for such a situation, or could you point me in the right direction?
Yeah, man!
You’ve basically described a classic case of carbon monoxide poisoning. Which was one of my favorite topics early in med school. (I even used it as a plot point in a novel I started writing. . . and then trunked because it had no plot.)
How does your blood carry oxygen?
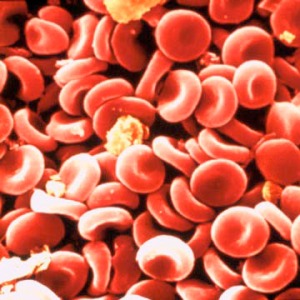
The red color of your blood is from all of the red blood cells floating in it.
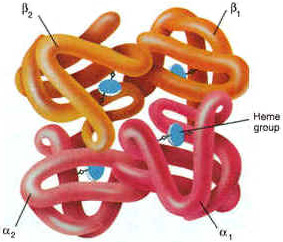
Red blood cells are really just sacks full of four-unit proteins called hemoglobin. This a representation of a hemoglobin protein:
Each of the parts of the protein has a little “pocket” that contains a unit of heme. I think heme is a truly awesome molecule, so I’m going to show its structure below. Notice the “Fe” in the middle. That’s the symbol for iron, and it’s very important to heme’s function. The other letters are also symbols for atoms; this tells you what the molecule is made of. C = Carbon, O = Oxygen, H = Hydrogen, N = Nitrogen.
Just look at it for a sec. Isn’t it gorgeous?
Quit geeking out, Doc.
Must I?
Yes. Get on with it.
Fine. Back to the point.
Heme has a good affinity for oxygen for the purposes of oxygen transport: It binds oxygen tightly enough to carry it around, but loosely enough to let it go when it arrives at its proper destination. (This “oxygen + hemoglobin” combination is called “oxyhemoglobin”.)
Enter carbon monoxide. Carbon monoxide is made up of one carbon atom and one oxygen atom. (The name tells you that, if you break it down.) Carbon monoxide also likes to bind to heme, in the same spot where oxygen likes to bind, right in the “pocket”.
Problem is, it binds WAY TOO TIGHTLY to the pocket, and is very difficult to release. (This “carbon monoxide + hemoglobin” combination is called “carboxyhemoglobin”. See, medical terminology isn’t THAT scary, is it?)
Carbon monoxide can not be used in the same way as oxygen. And it takes up all the heme groups that should be used to transport oxygen. And it doesn’t like to let go of heme once it’s grabbed on.
Long story short: Carbon monoxide interferes with proper oxygen transport, which seems to be the scenario you’ve described.
Fun with chemistry! Competition
Bear with me; we’re going conceptual. But I promise, it’s relevant to the subject matter.
First, picture a tank with a bunch of superballs bouncing around in it. That’s what goes on at a molecular level in most substances. A bunch of molecules bounce around, colliding with each other at random.
Now picture these superballs with extra appendages or depressions, three-dimensional fittings like puzzle pieces. If two balls hit each other in exactly the right orientation, they’ll attach together. The pairs, once formed, can also break apart spontaneously.
That’s how molecular events occur. And for these purposes, let’s say that these events are pretty common.
Now.
You have a population of red superballs with a particularly-shaped depression in them; they can only admit a certain shape of superball appendage upon collision. Now, two populations of superballs have that particular shape of appendage, one green population and one yellow population.
They can both attach to the red balls, but once they’re attached, the yellow balls don’t let go quite as easily as the green ones do. So, if you have equal populations of the two, eventually you’ll end up with more yellow-red pairs than green-red pairs. The yellow out-competes the green for attachment sites.
The only way you’ll get more green-red pairs than yellow-red pairs is by making sure the population of green balls FAR outnumbers the population of yellow balls.
Treating carbon monoxide poisoning, using molecular competition
This basically explains the basis of treatment for carbon monoxide poisoning. The red balls are the hemoglobin, with its particularly-shaped pocket. The green balls are oxygen, that can attach with some affinity. The yellow balls are carbon monoxide, which have a much higher affinity for the hemoglobin.
So, if you have a bunch of carbon monoxide bouncing around the system, oxygen will be out-competed for binding sites in the pockets of the available hemoglobin. The only way to correct this is by increasing the population of oxygen molecules as far as you can; putting in something like ten green balls for every one yellow one. Eventually your population will consist of mainly green-red pairs (oxyhemoglobin) and very few yellow-red pairs (carboxyhemoglobin).
So, the treatment for carbon monoxide poisoning is basically, saturate a person with oxygen in order to outcompete the carbon monoxide.
In answer to your question:
You can use carbon monoxide for your scenario if you want to; it seems to fit well. At that point you’d just turn up the oxygen on the bridge or find your character an oxygen mask, and out-compete the carbon monoxide.
Alternatively, you can propose another toxin that interferes somehow with the hemoglobin molecule, and then make up an antidote that (a) displaces the toxin from its site of interference or (b) binds up the toxin to keep it from getting to the site of interference.
Hope that helps! Thanks for writing!
Pictures:
http://www.sciencemuseum.org.uk/exhibitions/lifecycle/images/1-2-6-4-0-0-0-0-0-0-0.jpg
http://www.daviddarling.info/images/hemoglobin.jpg
http://omlc.ogi.edu/spectra/hemoglobin/hemestruct/heme-struct.gif
The contents of this site, such as text, graphics, images, and other material contained on the Site (“Content”) are for informational purposes only. The Content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this Site!
If you think you may have a medical emergency, call your doctor or 911 immediately. This blog does not recommend or endorse any specific tests, physicians, products, procedures, opinions, or other information that may be mentioned on the Site. Reliance on any information provided by this blog, or other visitors to the Site is solely at your own risk.
The Site may contain health- or medical-related materials that are sexually explicit. If you find these materials offensive, you may not want to use our Site. The Site and the Content are provided on an “as is” basis.
If you use this as if it were real medical information, I’ll fill all of your pockets with superballs. They will become very bouncy pockets.
—
 Reprinted with permission from Superballs, Pockets, and Fun With Awesome Molecules on How To Kill Your Imaginary Friends, by Dr. Grasshopper
Reprinted with permission from Superballs, Pockets, and Fun With Awesome Molecules on How To Kill Your Imaginary Friends, by Dr. Grasshopper
Dr. Grasshopper is a science fiction and fantasy author who is finishing up medical school and seeking residency in the field of internal medicine.